The Affordable and Effective Algicide!
Cupricide® is an economical product that provides effective control of a wide spectrum of filamentous and planktonic algae. It is suitable for use in irrigation canals, farm dams, ponds, ornamental lakes and potable water supplies.
The Need for Cupricide®!
In recent years, Australian water supplies have been increasingly threatened by nuisance algae growth caused by excessive amounts of nutrients entering water supplies. These nutrients, originating from agricultural or urban runoff, detergent wastes and/or waste water discharges can enhance the growth of aquatic algae often resulting in unsightly and unmanageable algal blooms. Under serious infestation, algae may accumulate on the surface forming a thick scum. Such algal blooms are not only aesthetically displeasing and detrimental to the intended use of the water but may also present serious health hazards to both humans and animals, e.g., blue green algae. Algae contamination may discolour water, create unpleasant tastes and odours and even impart a distasteful flavour to fish. In addition, once algae die and starts to decompose, the process may deplete dissolved oxygen levels in the water thereby threatening fish populations.
 Agmin’s Cupricide® is an economically viable way to control algae. Supplied in an easy to use (liquid) form, Cupricide® effectively controls the blooming of a broad spectrum of planktonic (suspended) and filamentous (mat forming) algae including Chara, Spirogyra, Cladophora, Vaucheria, Ulothrix, Microcystis, Anabaena, Nodularia and Oscillatoria. Cupricide® works by systemic action, penetrating the algal cells and inhibiting photosynthesis.
Agmin’s Cupricide® is an economically viable way to control algae. Supplied in an easy to use (liquid) form, Cupricide® effectively controls the blooming of a broad spectrum of planktonic (suspended) and filamentous (mat forming) algae including Chara, Spirogyra, Cladophora, Vaucheria, Ulothrix, Microcystis, Anabaena, Nodularia and Oscillatoria. Cupricide® works by systemic action, penetrating the algal cells and inhibiting photosynthesis.
The Benefits of a Chelated Copper Algicide
Agmin Chelates Pty Ltd Cupricide® is supplied as a mixed copper alkanolamine complex. In this form, Cupricide® provides maximum algicidal efficiency but with low toxicity to fish. The organic complexing agents used in Cupricide® ensure that the key active ingredient (Copper), remains in solution and does not precipitate out like some other copper algicides, namely copper sulphate. Although copper sulphate has been used extensively throughout Australia as an algicide, it has several drawbacks associated with its use. Firstly, copper sulphate is not algal specific - in fact it can be quite toxic to other aquatic life, e.g. fish when used at high concentrations. Secondly, if the water contains a high concentration of carbonate ions, the copper ions will preferentially combine with the carbonate ions and form an insoluble precipitate of copper carbonate. This precipitate sinks to the bottom of the water body where it forms a toxic slime. The formation of this precipitate also renders the copper essentially unavailable for the control of algae. To compensate, higher levels of copper sulphate are used which as stated above may seriously threaten other aquatic life. Finally, sulphate containing algicides also have the disadvantage that they combine with hydrogen ions in aqueous solution to form sulphuric acid which is highly corrosive. For the reasons stated above Cupricide® is a much more cost effective and efficient product for algae control than copper sulphate.
Background of Copper Algicides
In 1956, Maloney and Palmer found that copper ions in solution at a concentration of 0.1 – 0.5 ppm (mg/L) were capable of killing all algal cells, depending on the species tested. Not surprising, it has been found subsequently that there is a wide range of activity of copper ions against fresh water algae. The Table below summarises the main species of algae which have been tested against copper ions.
Table 1 |
|
Algae Species |
Lethal Cu Concentration |
|
Planktonic Algae |
0.1 – 0.5 ppm |
Filamentous Algae |
0.2 – 0.6 ppm |
Chara and Nitella |
0.4 – 0.8 ppm |
These early algicides were based on copper sulphate, which has several disadvantages:
- Carbonate in hard water precipitates copper ions
- Humic substances in natural waters reduce the availability of copper ions
- Free copper ions may be toxic to some fish species
- Toxic sediments accumulate on the bottom of the water body
Based on this historical background, Agmin Chelates Pty Ltd has developed a new stable copper-based algicide, Cupricide®, which overcomes the inherent disadvantages of copper sulphate, but displays high activity to the above range of algae.
Background of Cupricide®
Based on the fundamental principles of photosynthesis, we believe that Cupricide® is a potent inhibitor of photophosphorylation in the chloroplast cells of algae.
Photosynthesis is a chemical process used by algae in converting water, carbon dioxide and sunlight into oxygen and carbohydrates (such as sugar, starch, cellulose) – Refer Figure 1 (below).
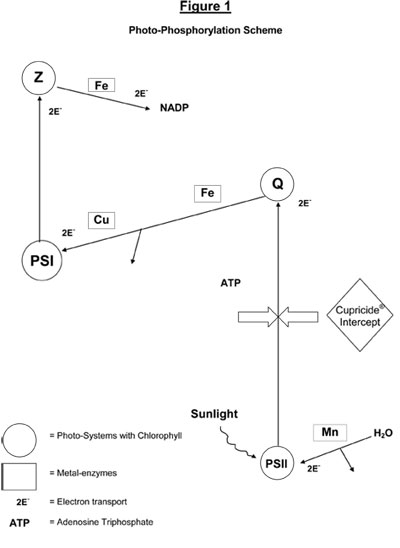
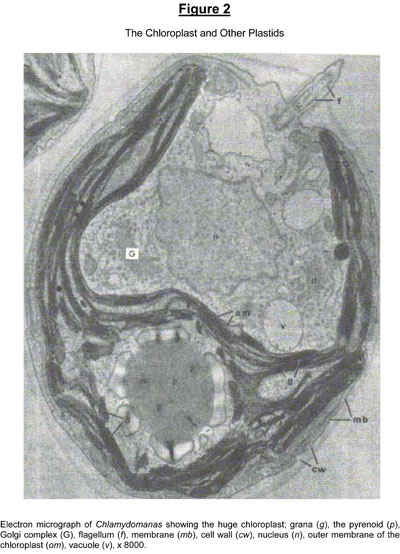
The reactions involved in photophosphorylation take place mainly in the “thylakoid membranes”, which are part of the granular structures contained within the walls of green chloroplasts. - Refer Figure 2(below).
Sunlight, a form of energy, is collected by chlorophyll molecules in the thylakoid membrane and is used to drive a complex sequence of reactions, involving electron transport between molecules, including water, oxygen, hydrogen ions, several proteins and quinones. Some of these proteins include ferredoxin (contains iron) plastocyanine (contains copper), cytochromes (contains iron) as well as plastoquinone. From laboratory studies of photosynthesis on isolated cells of chloroplasts, it is clear that interruption of any stage in the sequence of photophosphorylation will destroy the ability of the algal cell to photosynthesise and to survive.
Cupricide® has been found to destroy the thylakoid membrane, causing loss of chlorophyll and breakdown in the Photosytem II necessary to harvest sunlight. As a result, the algal cell ceases to function and literally shuts-down to die within a few days. The following schematic diagram (Figure 1) shows the reaction stage, when CUPRICIDE intercepts the electron transport from Photosystem II (chlorophyll-protein complex) to the high-energy intermediate electron carrier, Q.
Causes And Effects Of Excessive Nuisance Growth Of Aquatic Algae
Worldwide water supplies are threatened by excessive nutrients entering water supplies through agricultural runoff, wastewater discharges, detergent wastes, septic tank seepage and lawn and garden runoffs. Water containing excessive nutrients support dynamic growth of aquatic algae which interfere with intended uses of the water and sometimes presents health hazards to humans and animals. Algae are primitive plants which have no true leaves, stems or root systems.
Blue-green algae may cause illness, sometimes fatalities, in pets, livestock and wildlife. Exposure to or ingestion of blue-green algae may also cause a variety of discomforts in humans. Algae contamination of drinking water may discolour the water and create unpleasant tastes and odours. Excessive algae growth may also impart distasteful flavour to fish.
Decomposition of algae may deplete dissolved oxygen in bodies of water and kill fish. Excessive algae may block the intake of water from ponds and irrigation systems. Algae in wastewater oxidation ponds may elevate solids contents and biological oxygen demand. Scenic lakes, ponds, lagoons and shorelines may become unsightly with excessive algae growth.
Copper sulphate has been used extensively to control algae in many settings and to control parasites in fish aquaculture, but is has several disadvantages. Large quantities of copper sulphate are frequently necessary to satisfy the copper demand of a body of water caused by carbonate ions, which have an affinity for copper ions that precipitate out of the water. Also, copper ions that are not chelated may be very toxic to fish. The toxicity of copper sulphate varies with water hardness and is greater in soft water. Copper sulphate solution is unstable in sunlight and warm temperatures.
Cupricide® algicide combines the copper ions with organic complexing/chelating agents known as ethanolamines in order to eliminate the precipitation problem of copper sulphate and to render the copper less toxic to fish. Cupricide®, unlike copper sulphate, is not corrosive to equipment. It also has a long shelf life.
Cupricide® is effective in a wide range of fresh water systems. It works with systemic action by preventing photosynthesis within the chloroplasts of algal cells. The complex copper in Cupricide® is longer lasting than copper sulphate solutions and remains in solution for extended control. It provides economical and effective control of a broad spectrum of filamentous and planktonic algae.
Cupricide® is most effective when it is applied in bright, early morning sunlight under calm conditions when water temperature is at least 150C. Apply at the first sign of an algae bloom, if possible. Algae control will occur in 3 to 7 days following application. Re-apply when regrowth appears. Water treated with Cupricide® may be used for swimming, fishing, watering livestock and for irrigating turf, fairways, putting greens and ornamental plants immediately after treatment.

Algae makes a lake or water area look unsightly and almost unusable depending on the level of infestation.
Directions for Use
For most effective results, Cupricide® should be applied at the first signs of algal bloom. Apply Cupricide® under calm, sunny conditions when the water temperature is at least 15oC. Floating algae mats should be broken up either before spraying or during the application. Shore line areas should be sprayed first to avoid trapping fish.
As the algae die and decompose, dissolved oxygen levels in the water will be depleted. Thus when treating heavy infestations, treat only 1/3 to 1/2 of the water body at a time. This allows the oxygen levels to recover and prevents fish suffocation. The remaining water should be treated 14 days after the initial application.
For more specific instructions on Cupricide® use and safety precautions, please refer to the product label and material safety data sheet.
Types of Algae
 Planktonic Algae.
Also known as suspended algae this type includes forms such as Microcyctis, Oscillatoria, Anabaena, Euglena, Aphanizomenon. They are generally found suspended in the upper 1 - 1.5m of water imparting a green or brown colour to the water. Some species may be toxic to livestock and wildlife or impart a foul taste to fish. For effective control of planktonic algae, apply Cupricide® to the upper 1 - 1.25m of the water with application rates specified on the label.
Planktonic Algae.
Also known as suspended algae this type includes forms such as Microcyctis, Oscillatoria, Anabaena, Euglena, Aphanizomenon. They are generally found suspended in the upper 1 - 1.5m of water imparting a green or brown colour to the water. Some species may be toxic to livestock and wildlife or impart a foul taste to fish. For effective control of planktonic algae, apply Cupricide® to the upper 1 - 1.25m of the water with application rates specified on the label.
Filamentous Algae. Filamentous algae such as Spirogyra, Cladophora, Chlorella, or Oedogonium typically form greenish scum mats on the water surface or appear as a furry growth on logs or rocks. For effective control with Cupricide®, large algae mats should be broken up prior to Cupricide® application. Only the upper 1 - 1.5m of the water body needs to be treated using the specified application rates (see label for details).
Filamentous algae such as Spirogyra, Cladophora, Chlorella, or Oedogonium typically form greenish scum mats on the water surface or appear as a furry growth on logs or rocks. For effective control with Cupricide®, large algae mats should be broken up prior to Cupricide® application. Only the upper 1 - 1.5m of the water body needs to be treated using the specified application rates (see label for details).
 Chara and Nitella. These type of algae are most prevalent in hard water. The may be green, yellow or grey in colour. For best treatment results it is important to apply Cupricide® early in the season using application rates specified on the label.
Chara and Nitella. These type of algae are most prevalent in hard water. The may be green, yellow or grey in colour. For best treatment results it is important to apply Cupricide® early in the season using application rates specified on the label.
For more information please view our Cupricide News Letters.

